Elon Musk's brain implant company Neuralink expands to UK and begins human clinical trials

??????? We're launching a clinical study in Great Britain!
— Neuralink (@neuralink) July 31, 2025
In partnership with @uclh and @NewcastleHosps , we're exploring how our brain-computer interface can restore digital autonomy to individuals with severe paralysis by enabling them to control devices with their thoughts. pic.twitter.com/gajGwIgm5a
UCLH to evaluate safety and functionality of Neuralink's Brain-Computer Interface (BCI) technology : University College London Hospitals NHS Foundation Trust
https://www.uclh.nhs.uk/news/uclh-evaluate-safety-and-functionality-neuralinks-brain-computer-interface-bci-technology
Neuralink is a San Francisco-based brain-machine interface development company that is expected to receive approval for human clinical trials and begin recruiting subjects in 2023. In January 2024, the company announced the success of its first human clinical trial, and Musk announced that Neuralink's first product would be called 'Telepathy.'
Elon Musk's brain modification company 'Neuralink' succeeds in first human clinical trial, allowing smartphones and PCs to be controlled just by thinking, product name 'Telepathy' - GIGAZINE
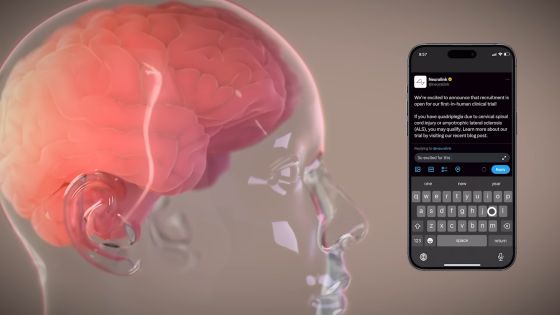
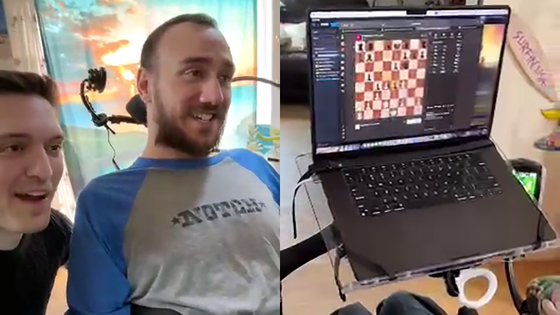
The article below clearly shows how a paralyzed man who underwent Neuralink's human clinical trials actually plays games just by thinking.
A paralyzed man undergoing Neuralink's human clinical trials releases a movie in which he controls a PC and plays chess just by thinking - GIGAZINE
Neuralink has announced that it will begin human clinical trials in the UK in partnership with University College London Hospitals (UCLH) and Newcastle Hospital. Neuralink is currently conducting human clinical trials in Canada and the United Arab Emirates, but the UK will be its first in Europe. Neuralink's human clinical trials have been approved by the UK Medicines and Healthcare products Regulatory Agency (MERA).
'UCLH has been selected as the UK lead site for GB-PRIME, a clinical investigation into Neuralink's brain-computer interface,' said University College London Hospitals in a press release. 'The launch of GB-PRIME at UCLH marks an important step towards advancing a technology that has the potential to transform the lives of people with neurological disorders around the world.' William Muirhead, principal investigator of GB-PRIME, added, 'This research is a commitment to developing pioneering treatments that will restore function, independence, and the ability to communicate to patients with severe neurological disorders. UCLH is proud to be at the forefront of this field, combining clinical excellence with cutting-edge neuroscience to deliver meaningful innovations to patient care.'

At the time of writing, up to seven British people with neurological conditions who are unable to walk or manually operate a computer or smartphone are expected to participate in the clinical trial.
Neuralink is not the only company aiming to realize a brain-computer interface by implanting electrodes in the brain. In June 2025, a rival startup, Paradromics , announced that it had successfully implanted a device in a human for the first time. Cortec, based in Freiburg, Germany, also announced the implantation of the first German-made brain-computer interface in a human on July 29, 2025, changing the way clinical trials are conducted around the world.
Related Posts:
in Science, Posted by log1e_dh