Paradromics, a competitor to Elon Musk's Neuralink, has successfully implanted the first brain-computer interface

On June 2, 2025,
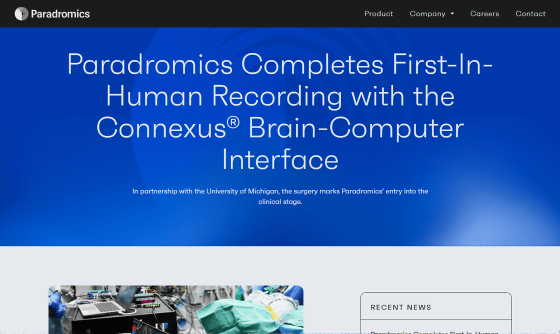
Paradromics Completes First-In-Human Recording with the Connexus® Brain-Computer Interface
https://www.paradromics.com/news/paradromics-completes-first-in-human-recording-with-the-connexus-brain-computer-interface
Neuralink competitor Paradromics completes first human implant
https://www.cnbc.com/2025/06/02/neuralink-paradromics-human-implant.html
Neuralink Rival Paradromics Successfully Implants Connexus Brain-Computer Interface (BCI) in Epilepsy Patient for the First Time - TechEBlog
https://www.techeblog.com/paradromics-connexus-brain-computer-interface-bci/
On May 14, 2025, Paradromics, led by an interdisciplinary team of neurosurgeons and engineers from the University of Michigan, successfully implanted its BCI device, Connexus , into a human brain.
Paradromics' BCI platform records neural activity at the individual neuron level and uses artificial intelligence to convert brain signals into actionable outputs. Connexus is designed to restore communication abilities to people with severe motor disabilities caused by amyotrophic lateral sclerosis (ALS) , brainstem stroke , spinal cord injury, and other conditions.
The surgical procedure was performed on a patient undergoing neurosurgery to treat epilepsy. Dr. Matthew Wilsey, a neurosurgeon and biomedical engineer at the University of Michigan, and his colleagues demonstrated that the Connexus could be surgically implanted into the patient's brain, record electrical signals from the brain, and then be removed without causing any damage within 20 minutes.
On the left is Dr. Steven Liu, Chief Medical Officer of Paradromics, and on the right is Dr. Wilsey.

Paradromics claims that this procedure demonstrates that Connexus can be safely implanted in the body and used to record neural activity. If approved by regulators, Paradromics plans to begin clinical trials in 2025 to study the long-term safety and use in humans.
'This surgery marks an important turning point for Paradromics,' said Dr. Matt Angle, founder and CEO of Paradromics. 'We are now a clinical-stage company. Based on our preclinical research, we have recognized that we have developed a world-class BCI platform. By moving to human surgery and recording, we are getting closer to applying this neurotechnology to patients.'
'Leveraging advanced BCI recording platforms like Connexus BCI, my lab is developing the next generation of speech and motor assist devices,' said Dr. Wilsey. 'This research is a major step towards providing treatment to patients with severe unmet medical needs.'

Meanwhile, Neuralink announced on June 2, 2025 that it had raised $650 million (about 93 billion yen) in its latest funding round. In a statement, Neuralink said, 'This funding will enable us to bring our technology to more people, help people with underserved medical needs gain independence, and push the boundaries of what's possible with brain interfaces.'
Musk's Neuralink raises $650 million in latest funding as clinical trials begin | Reuters
https://www.reuters.com/business/healthcare-pharmaceuticals/musks-neuralink-raises-650-million-latest-funding-round-2025-06-02/
Related Posts: