Gene therapy shows promising results in significantly slowing the progression of Huntington's disease, an incurable disease that ultimately leaves patients bedridden
uniQure Announces Positive Topline Results from Pivotal Phase I/II Study of AMT-130 in Patients with Huntington's Disease | uniQure BV
https://uniqure.gcs-web.com/news-releases/news-release-details/uniqure-announces-positive-topline-results-pivotal-phase-iii

New gene therapy slows progression of Huntington's disease by 75% | UK News | Sky News
https://news.sky.com/story/new-gene-therapy-slows-progression-of-huntingtons-disease-by-75-13437292
Huntington's disease is a progressive genetic disorder that causes psychiatric symptoms such as chorea, which is an involuntary movement of the limbs and face, cognitive decline, delusions, depression, and personality changes. The disease typically begins between the ages of 30 and 50, but progresses gradually, eventually leading to unsteady walking and difficulty swallowing, eventually leading to severe dementia and bedriddenness.
Huntington's disease is caused by a mutation in the first exon of the huntingtin gene (HTT), a multifunctional gene that encodes a protein called huntingtin . It is inherited with a 50% probability regardless of gender. There are approximately 75,000 patients across the United States and Europe, but there is still no treatment that can cure Huntington's disease, delay its onset, or slow its progression.
uniQure is a biotechnology company developing a gene therapy for Huntington's disease called AMT-130. AMT-130 consists of an adeno-associated virus vector incorporating an artificial microRNA tailored to suppress the huntingtin gene, thereby inhibiting the production of mutant huntingtin.
The following video explains what kind of gene therapy drug AMT-130 is.
AMT-130 Administration - YouTube
AMT-130 is a gene therapy drug developed to reduce levels of abnormal huntingtin protein in the brain and slow the progression of Huntington's disease.
The targets are areas in the center of the cerebrum, such as
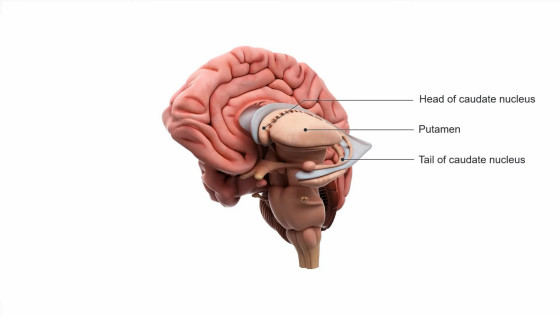
AMT-130 is delivered to these target areas using tiny
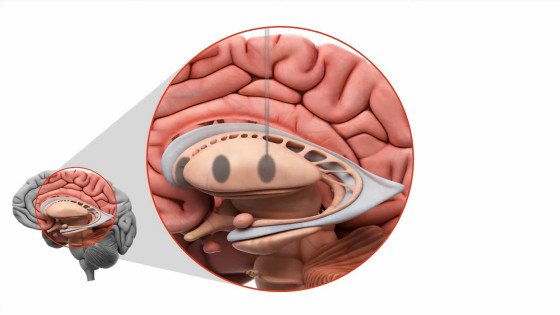
AMT-130 is delivered directly to the affected area of the brain, and is said to have long-lasting effects with just one dose.
On September 24, 2025, uniQure announced positive data from a Phase I/II clinical trial of AMT-130. A total of 29 patients were administered AMT-130 in the clinical trial, and disease progression was compared between patients in a control group who did not receive AMT-130 and those in a control group based on the Composite Unified Huntington's Disease Rating Scale (cUHDRS) and Total Functional Capacity (TFC).
Over a 36-month follow-up period, patients receiving high-dose AMT-130 experienced a 75% slowing of disease progression as measured by the cUHDRS and a 60% slowing of disease progression as measured by the TFC. Furthermore, significant delays in disease progression were also observed in measures of cognitive and motor function, including the Symbol Digit Modalities Test (SDMT), the Stroop Word Reading Test (SWRT), and the Total Motor Score (TMS), compared to the control group.
Professor Ed Wilde, of the Huntington's Disease Centre at University College London, who led the UK clinical trial, commented, 'Based on these results, AMT-130 is likely to become the first approved treatment to slow the progression of Huntington's disease. This is truly world-changing.' 'My participating patients have shown stability over time, which is unusual for Huntington's disease,' he said. 'If treated early, they may be able to live a high quality of life for years or even decades, or even be free of the disease completely.'
Related Posts: